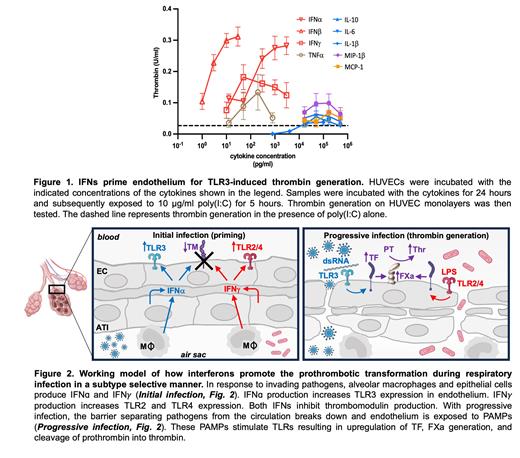
Cytokine storm occurs with respiratory infection and is associated with microvascular thrombosis. However, the cytokines responsible for promoting thrombus formation in the pulmonary microvasculature and the mechanisms by which they act are largely unknown. In its initial response to invading viruses or bacteria, pulmonary epithelium and macrophages release a variety of cytokines into the pulmonary circulation. With progressive invasion, the fragile barrier between alveolus and pulmonary capillaries breaks down and endothelial cells are exposed to pathogen-associated molecular patterns (PAMPs), which stimulate endothelial toll-like receptors (TLRs). To identify the cytokines that stimulate endothelial prothrombotic activity, we screened MIP-1β, MCP-1, IL-10, IL-6, IL-1β, TNFα, IFNα, IFNβ, and IFNγ for their ability to synergize with PAMPs in order to stimulate thrombin generation on endothelium. Dose curves showed that IFNs and TNFα were ~1000-fold more potent than the other cytokines at priming endothelium for thrombin generation induced by poly(I:C), a synthetic PAMP that stimulates TLR3 ( Fig. 1). Anti-tissue factor (TF) antibody completely attenuated thrombin generation induced by either IFNα or IFNγ exposure followed by poly(I:C) stimulation. Evaluation of TF expression and FXa generation showed that although exposure of endothelium to IFNs did not itself enhance TF expression or activity, IFNs substantially augmented TF expression and activity in response to poly(I:C). In addition, IFNα and IFNγ significantly inhibited thrombomodulin expression, even in the absence of poly(I:C). To evaluate whether or not IFNs enhance thrombin generation in endothelium stimulated through TLRs other than TLR3, we exposed endothelium to IFNα or IFNγ, followed by stimulation with different TLR-selective agonists. IFNγ exposure enhanced thrombin generation on endothelium in response to the TLR1/2 agonist PAM3CSK4, but IFNα did not have a significant effect, indicating the specificity of coupling to a particular IFN subtype. In contrast, the stimulation of endothelial thrombin generation by the TLR3 agonists, poly(I:C) and poly(A:U), was more potently enhanced by IFNα compared to IFNγ. Stimulation of thrombin generation by TLR4 agonists, including LPS and CRX-527, was significantly enhanced by IFNγ, but not IFNα. This coupling of IFNα with TLR3 agonists is consistent with the known association of viral infection with IFNα stimulation and TLR3 activation. Coupling of IFNγ with TLR2/4 agonists reflects a previously identified association of bacterial infection with both IFNγ stimulation and TLR 2 and 4 activation. Evaluation of conditioned media from pulmonary epithelial cells showed that media from inflamed pulmonary epithelial cells, but not quiescent epithelium, primed endothelium for substantial thrombin generation in response to poly(I:C). Anti-IFNα antibodies blocked the priming effect of inflamed condition media. These studies show that endogenous IFNs elaborated by pulmonary epithelium prime endothelium for TLR-mediated thrombin generation. These results show that IFNs are both necessary and sufficient to enhance TLR-mediated endothelial thrombin generation and provide a new framework for understanding the role of IFNs in cytokine-related thrombus formation ( Fig. 2). This model indicates that endothelial cells possess a verification system wherein a robust thrombotic response is only initiated if the type of IFN released by alveolar macrophages and epithelium is consistent with the type of TLR sensed by the endothelium. These observations provide a mechanistic basis for microvascular thrombosis in cytokine storm and raise the possibility of targeting interferons to prevent thrombotic complications in this setting.
Disclosures
Eaton:Thermofisher: Current Employment. Flaumenhaft:Versiti Blood Research Center: Membership on an entity's Board of Directors or advisory committees; Function Therapeutics: Membership on an entity's Board of Directors or advisory committees; Platelet Diagnostics: Consultancy, Current equity holder in private company, Patents & Royalties; Cleveland KUH- Training network: Honoraria, Membership on an entity's Board of Directors or advisory committees; Xap Therapeutics: Consultancy; Porosome Therapeutics: Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal